Every day, Michael Shiferaw is reminded of the profound threat cancer poses to individuals and communities worldwide as another diagnosis is made. In 2025, the United States alone is projected to see over two million new cancer cases, with more than six hundred thousand deaths anticipated. Globally, the cancer burden is expected to rise sharply. Radiation therapy remains a cornerstone treatment, used to eliminate malignant cells by targeting tumors. Yet some cells develop resistance to radiation, repopulating tumors and leading to poor patient outcomes. Fortunately, researchers like Michael are at the forefront of research efforts to improve radiation therapy and develop alternative treatments: advances critical to enhancing efficacy and survival rates.
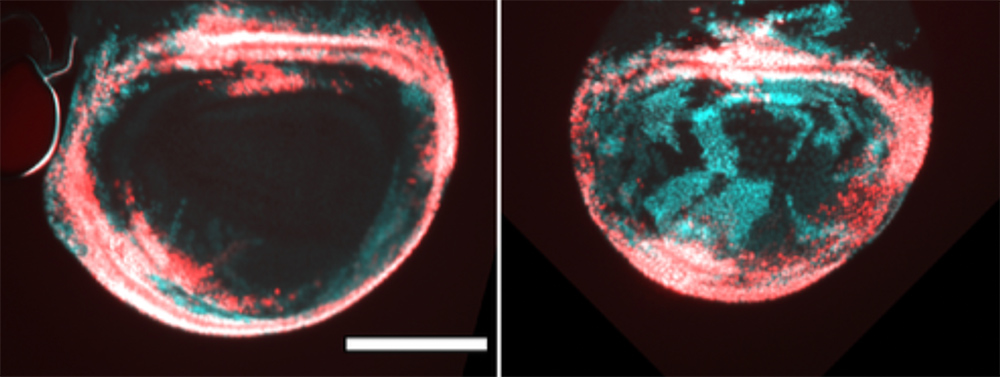
Epithelial cells without radiation (left) and with radiation (right). The left image shows cells marked with a red fluorescent protein, indicating their normal location within the tissue. The right image reveals the impact of radiation: cells with green/cyan fluorescence emerge, showing they have changed their fate/normal state, and it’s clear these cells have migrated inward to repair the damage caused by radiation. This is particularly concerning because if tumors targeted with radiation can recover the damaged cells that were killed, it is critical to prevent this from happening.
At the heart of Michael’s work is a pivotal question: Which genes within these cells shift their activity to help tumors regenerate after radiation therapy? By identifying these genes, Michael and his collaborators aim to unravel how certain cancer cells rebound despite aggressive treatments. His findings, recently showcased at conferences like TAGC24 in Washington, D.C., and Dros25 in San Diego, highlight the potential to disrupt resistance mechanisms. “If we can pinpoint and validate these genes,” he explains, “it opens the door to developing targeted drugs or inhibitors. This could make radiation therapy far more effective.”
Michael’s interdisciplinary expertise, spanning biochemistry, molecular and cell biology, and machine learning, fuels his innovative approach. Currently based at the University of Colorado Boulder, a hub for groundbreaking biological and scientific research led by Nobel laureates like Tom Cech and Jennifer Doudna, he thrives in an environment of collaboration and discovery. “It’s humbling to work alongside scientists pushing the boundaries of what’s possible,” he says. Reflecting on his journey, he adds, “Everyone I admire once stood where I am now. We all start somewhere. I’m excited to bring my best every day and contribute to this field.”
Under the mentorship of Dr. Tin Tin Su, a trailblazer in radiation biology, Michael is building on a legacy of transformative research. Dr. Su’s work spans decades, from pioneering studies on how ionizing radiation damages cells to co-founding the biotech startup Suvica, which developed the novel cancer drug SVC112. Now, Michael is advancing the mission by probing the molecular drivers of therapy-resistant tumors. His goal: to uncover new targets that could tip the scales in favor of patients.
Bridging computation and biology, Michael also designs tools to streamline lab workflows and analyze complex datasets. “Machine learning isn’t just about automation—it’s about spotting patterns humans might miss,” he says. He predicts that within five years, these tools will accelerate discoveries, cut costs, and reveal insights into diseases that traditional methods cannot. “The synergy between biology and AI will be transformative, creating jobs and delivering real value to society,” he adds.
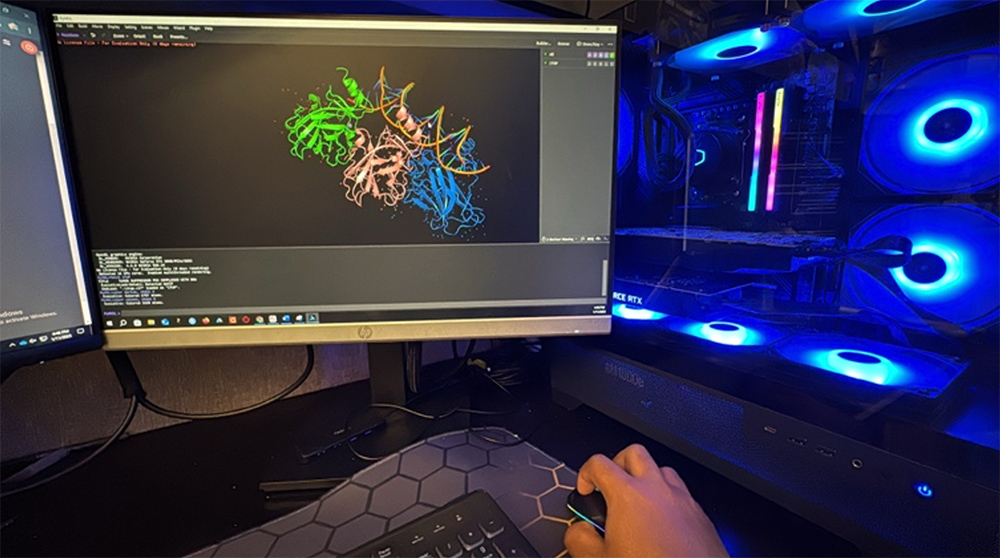
Computational analysis of proteins identified through genetic screens.
Recognized with awards like the NSF Rising Scientist Award and an NIH-supported grant, Michael balances ambition with a commitment to accessibility. “Science thrives when knowledge is shared openly,” he emphasizes. By mentoring others and fostering collaboration, he aims to create ripples of progress beyond his own lab. “Many diseases we struggle with today, like cancer, will become far more manageable as we deepen our understanding of cell and molecular biology,” he says. “Solving these problems requires starting from first principles: asking what we truly know and building from there.”
This philosophy has already led Michael to discover previously overlooked genes linked to tumor regeneration. While further testing is underway to validate their mechanisms, there’s pragmatic optimism that his work could improve radiation therapy outcomes. For Michael, the path forward is clear: “I’m determined to do my part. Every breakthrough, brings us closer to a future where cancer is no longer a devastating diagnosis.”