Karmanos Cancer Institute announced the launch of a new clinical trials study information portal (SIP) on Karmanos.org. This portal was launched on Monday, November 9, 2020.
The SIP website, which is integrated with OnCore Clinical Trials Management System, is used by medical professionals, researchers and the general public to search clinical trials recruiting at Karmanos. The site allows users to easily search, sort and navigate through the many clinical trials available at Karmanos Cancer Institute.
Features include:
- Principal investigator profiles
- Eligibility criteria from clinicaltrials.gov
- List of studies on related-physician profiles
- Location maps and phone numbers of study sites
- Search fields including cancer multidisciplinary team/type, investigational device, NCT ID and multiple search terms
Mobile-friendly design (Note: The Karmanos Clinical Trials Mobile App will still be available).
Additional features are expected to go live in the coming months. To view the new portal, visit karmanos.org/opentrials.
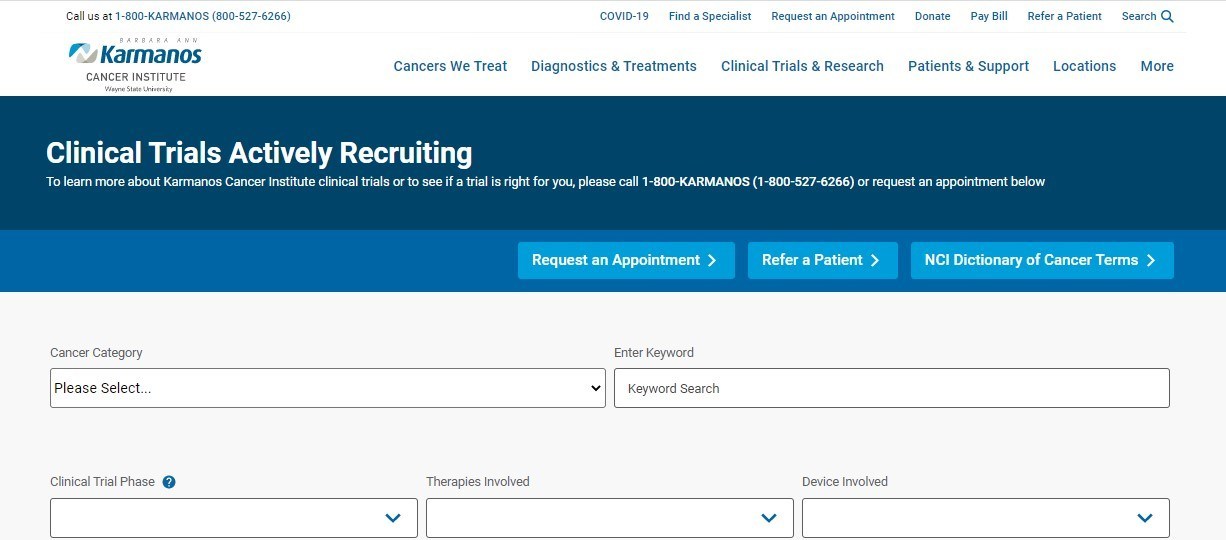
The new clinical trials study information portal (SIP) allows users to easily search, sort and navigate through the many clinical trials available at Karmanos Cancer Institute.
“The launch of the clinical trials study information portal allows us to better serve our patients and creates greater ease for our physician partners and researchers as they explore Karmanos’ clinical trial offerings. We are proud to offer more than 800 clinical trials to give patients access to tomorrow’s care today. By creating an easier way to organize information about these trials, we can ensure that these treatments reach all patients who are eligible,” said Gerold Bepler, M.D., Ph.D., president and CEO of Karmanos Cancer Institute.
The Barbara Ann Karmanos Cancer Institute has one of the largest and best clinical trial programs in the United States, giving patients better access to new cancer treatments. Our patients have access to more than 250 promising new cancer treatments often available only at Karmanos Cancer Institute. Karmanos has patients actively participating in more than 800 clinical trials, which are developed and sponsored by our own physicians and researchers, major pharmaceutical companies or national cooperative group programs funded by the National Cancer Institute (NCI).
This allows us to offer patients treatments that are often not available anywhere else. In fact, one-third of all new cancer drugs were developed with our participation in trials. As the state’s only hospital focused solely on cancer, we take our research role seriously while also delivering care with the utmost compassion and understanding. With our long-term partnership with the Wayne State University School of Medicine, we are committed to promoting excellence in cancer research, education and clinical care. To speed treatments from the laboratory to the patient’s bedside, our partnership represents a synergistic collaboration between the laboratory scientists, cancer experts and clinicians who interact directly with patients. Together, we have conducted research that has contributed substantially to therapeutic breakthroughs in cancer and that continues to define new standards of care.





